Our research is supported by dedicated science & technology platforms:
Research Overview
Research is the driving force of Nerviano Medical Sciences. With a long-standing expertise in R&D, we have a proven ability to conduct innovative research and advance molecules from discovery through clinical development to registration. Our work focuses on key biological targets and mechanisms that drive the onset and progression of cancer. By exploring novel pathways, identifying innovative drug targets, and developing both first- and best-in-class therapies, we aim to combat treatment resistance and improve patient outcomes.
Cell Cycle & DNA Repair
Uncontrolled cell proliferation and defective DNA repair are hallmarks of cancer. By targeting key cell cycle proteins like CDKs, PLK1, and MPS1, and exploiting synthetic lethality in DNA repair pathways such as BRCA1/2 and PARP, we aim to inhibit cancer growth while preserving healthy cells. Our second-generation non-trapping, brain penetrant PARP1 inhibitor holds the potential to be first and best-in-class.
Tumor Metabolism & Protein Homeostasis
Cancer cells often rely on altered metabolism and protein regulation to sustain their growth and survive under stress. Our research focuses on identifying and targeting these vulnerabilities, such as the Unfolded Protein Response (UPR) and its key regulator PERK and GCN2, to induce cancer cell death through proteotoxicity, particularly in diseases like Multiple Myeloma and Acute Myeloid Leukemia.
Immuno-Oncology
While immunotherapy has revolutionized cancer treatment, we are exploring new ways to enhance immune responses against cancer. Our research focuses on discovering small molecules that either stimulate immune cells to attack tumors or make cancer cells more recognizable to the immune system. Small molecules offer unique advantages, including oral administration and the potential for combination with existing therapies to improve efficacy.
Small Molecule Platform:
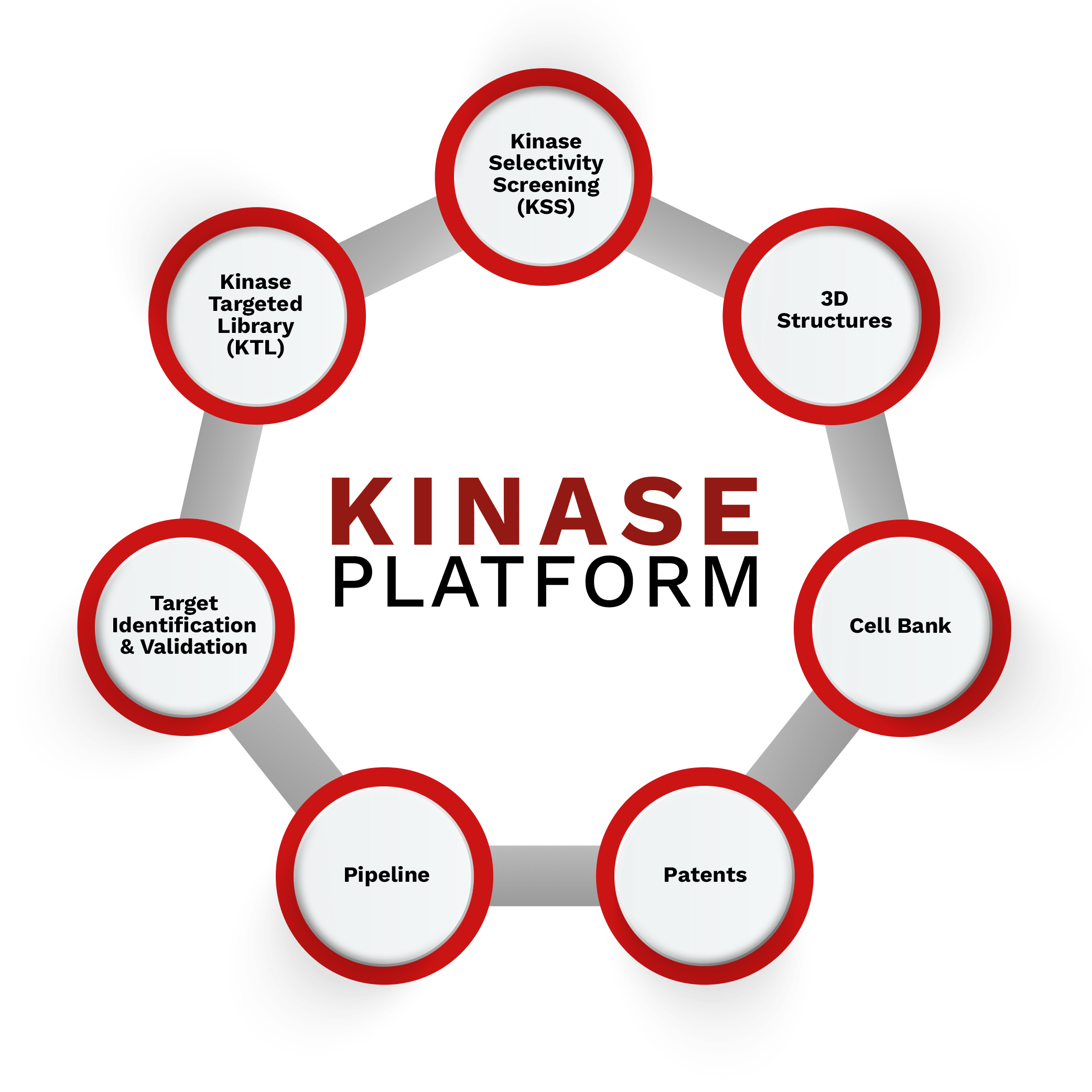
Kinase Platform
Kinases are a crucial class of pharmaceutical targets, valued for their broad biological relevance and druggability by small molecules. As pioneers in recognizing the importance of Kinases for personalized anticancer therapies, Nerviano Medical Sciences has built a dedicated Kinase Platform. This specialized infrastructure integrates advanced chemistry and biology approaches to target various Kinase families.
Our Kinase Platform has successfully delivered potent and selective inhibitors, several of which are either approved or in clinical development, reinforcing our commitment to advancing targeted cancer therapies.
We remain dedicated to leveraging our deep expertise in Kinases to advance and continuously expand our broad portfolio of preclinical and clinical Kinase inhibitor programs. Focused on the key areas of Oncology and Immuno-oncology, we are committed to driving innovation and developing next-generation therapies that address unmet medical needs.
Kinase as drug targets
Clinical Validation
Kinases regulate key intracellular pathways that become dysregulated in cancer, often referred to as the “hallmarks of cancer.” Currently, there are over 40 kinase-targeted drugs approved for a wide variety of solid and hematological tumors, with an impressive number of additional molecules in clinical development.
Unexplored Potential
Despite significant progress in the development of Kinase inhibitors over the past two decades, the full therapeutic potential of Kinase inhibition has yet to be fully realized. As our understanding of the physiological and pathological roles of the 500+ Kinase enzymes expand, new opportunities unlock innovative therapeutic avenues.
Medical Need and Emerging Role in Combination Therapies
Kinase inhibitors exemplify the success of targeted therapies, offering prolonged and often remarkable responses in patients selected for specific targets. However, drug resistance remains a clinical challenge, driving the urgency for next-generation inhibitors designed to overcome resistance mechanisms. Furthermore, cutting-edge research into combination therapies, to unlock the potential to amplify and extend the efficacy of Kinase inhibitors, offers new hope for more durable and comprehensive treatment strategies.
NMS Kinase Platform
A robust infrastructure of expertise, intellectual property, and advanced tools dedicated to Kinases. With over 20 years of integrated chemistry and biology approaches, our platform drives the rapid development of potent, selective Kinase inhibitors. It leverages an economy of scale to ensure the continuous delivery of first-in-class or best-in-class molecules, driving innovation across different tumor settings while evolving with the latest scientific and technological advancements.
Target identification and validation
Target Identification and Validation
We apply integrated genomics and proteomics profiling of cancer cell lines and tumor samples to uncover dysregulated networks and identify potential new therapeutic targets. Using advanced bioinformatics, including proprietary in-house tools, we mine both internal and external databases to pinpoint new genomic alterations involving Kinases in specific tumor contexts. Our large-scale gene silencing and phenotypic screenings across molecularly characterized cancer cell panels help identify synthetic lethality contexts and novel targets in select genetic backgrounds. These approaches have led to the discovery of several new targets at NMS, with promising first-in-class programs underway.
Kinase Targeted Libraries (KTL)
Our Kinase Targeted Library (KTL) is a proprietary collection of approximately 100,000 molecules, specifically designed to interact with Kinases. Synthesized entirely in-house, the library spans over 100 diverse chemical classes, offering broad coverage of the Kinase inhibitor chemical space. It serves as an ideal starting point for hit identification and structure-activity relationship (SAR) studies. The KTL is continuously expanded through crystallography and modeling approaches, which also enhance hit affinity and selectivity for specific targets. The KTL, along with an extended library for other purine-binding enzymes (PTL), provides a robust resource for hit discovery, with extensive patent protection and strong intellectual property positions.
Kinase Selectivity Screening (KSS)
Our Kinase Selectivity Screening (KSS) platform consists of a panel of over 100 automated biochemical Kinase assays, developed in-house at NMS. These assays evaluate the selectivity profiles of compounds and expand our knowledge of chemical interactions across different Kinases. KSS is instrumental in supporting Structure-Based Drug Design (SBDD) for Kinase inhibitors.
The enzyme proteins used in these screenings are entirely produced and characterized in-house, allowing for robust potency assessments (IC50) that are directly comparable across various targets. The panel is constantly updated with new, unexplored Kinase targets, providing opportunities for the discovery of new hits and leads across our diverse Kinase projects.
Our Kinase Selectivity Screening (KSS) platform consists of a panel of over 100 automated biochemical Kinase assays, developed in-house at NMS. These assays evaluate the selectivity profiles of compounds and expand our knowledge of chemical interactions across different Kinases. Over time, KSS has supported the discovery of more than 14,000 hits, providing critical insights that drive Structure-Based Drug Design (SBDD) for Kinase inhibitors.
The enzyme proteins used in these screenings are entirely produced and characterized in-house, allowing for robust potency assessments (IC50) that are directly comparable across various targets. The panel is constantly updated with new, unexplored Kinase targets, providing opportunities for the discovery of new hits and leads across our diverse Kinase projects.
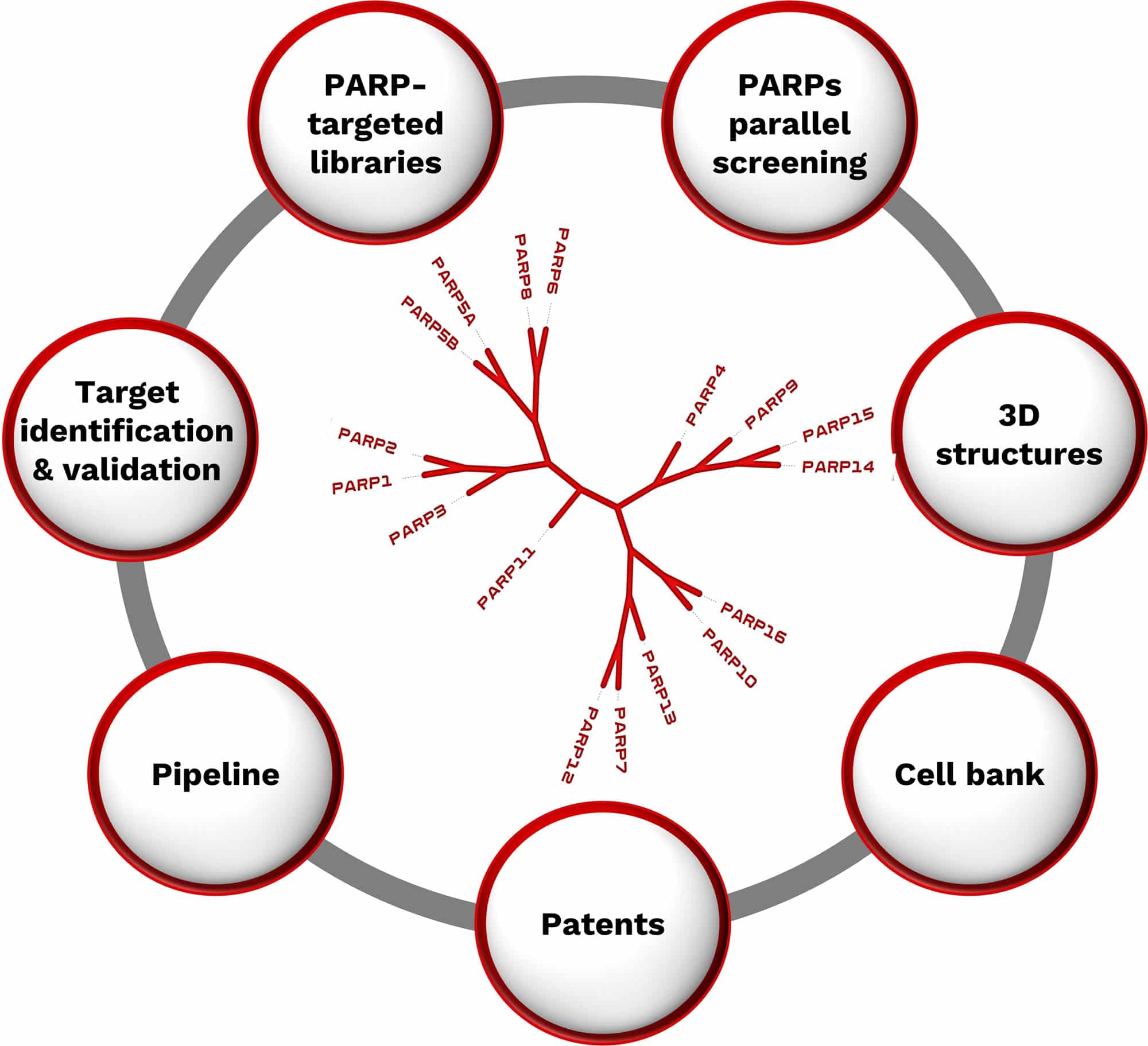
NAD+Binding Platform: PARP
A novel platform implemented based on PARP family (~17 enzymes) facilitating the rapid identification of compounds for novel target validation and potentially new drugs
Allows the identification of novel chemical matter exploiting the NAD binding pocket with the potential of expansion to other NAD-binding family enzymes
ADC Platform
NMS ADC integrated platform of payload linkers
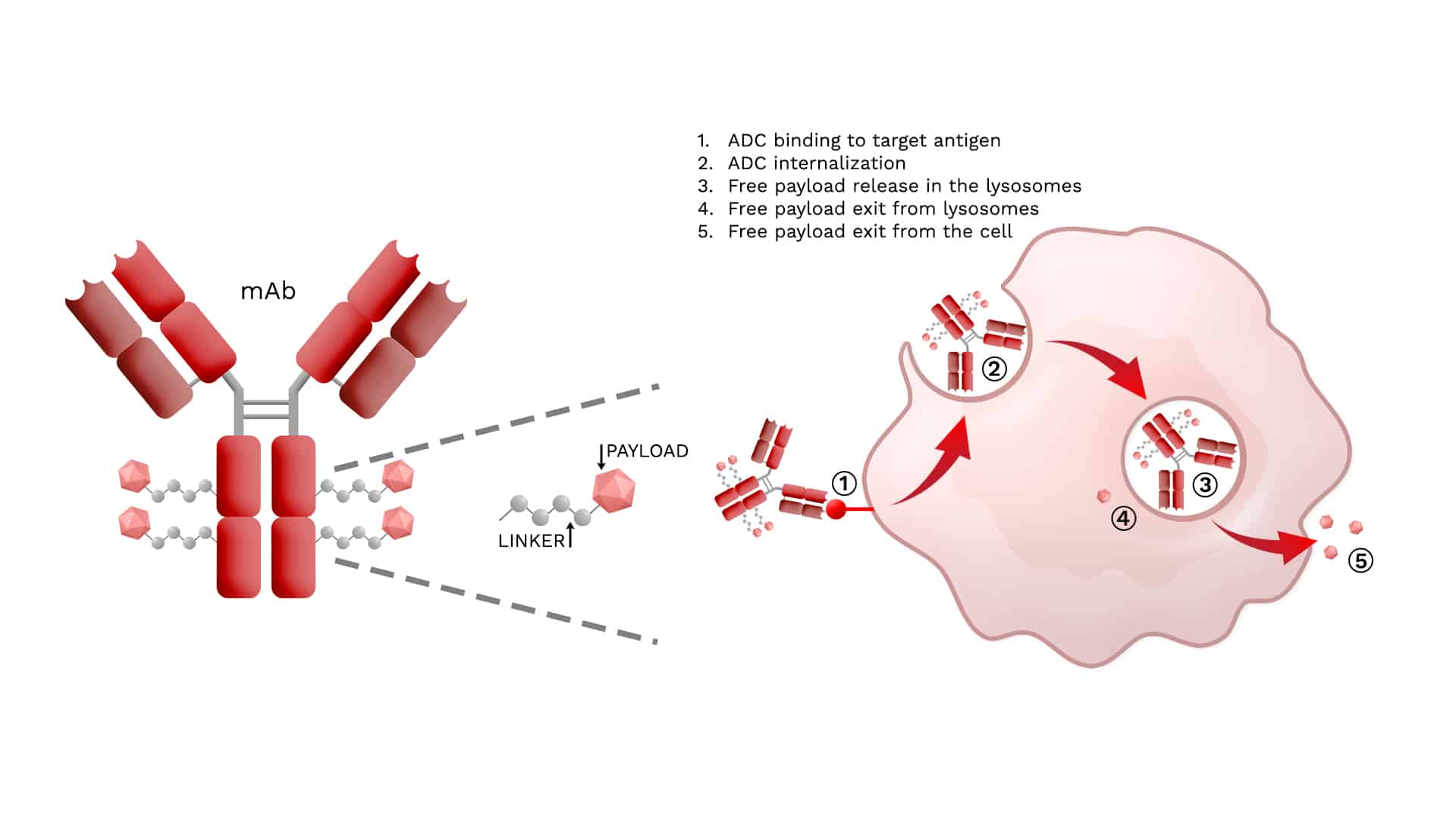
Antibody-Drug Conjugates (ADCs) are groundbreaking in the realm of targeted cancer therapy, marrying the specificity of monoclonal antibodies (mAbs) with the potent killing power of cytotoxic drugs. Their design leverages the precision of antibody targeting to deliver cytotoxic agents directly to cancer cells, thereby sparing normal, healthy cells from the toxic effects of chemotherapy. This approach enhances the efficacy of the drug and minimizes side effects, offering a promising treatment option for various types of cancer.
ADCs are a highly sophisticated pharmaceutical, made of three main components:
- Monoclonal antibody (mAb) – targets specific tumor antigens.
- Linker – connects the mAb to the cytotoxic drug.
- Payload – cytotoxic drug delivered specifically to cancer cells.
Combining the specificity of antibodies with the lethal potency of cytotoxic drugs paves the way for more effective and less toxic cancer therapies. The innovative design of ADCs represents a significant advancement in oncology, offering hope for more effective treatments with fewer side effects.
NMS is at the forefront of developing these cutting-edge therapies, contributing to the future of cancer treatment.
Nerviano Medical Sciences’ edge in ADC technology: integrating key research expertise to create next-generation ADCs
Developing fully functional ADCs is a sophisticated process that necessitates a multidisciplinary approach, integrating insights and techniques from molecular biology, chemistry, pharmacology, and bioengineering. NMS, with decades of expertise, innovates and integrates the various components and processes involved in ADC development to address emerging limitations in the field, including chemoresistance and limited therapeutic window.
NMS portfolio includes optimized duocarmycin (NMS-P945), anthracycline, and targeted payloads tailored to effectively treat diverse tumors with enhanced specificity. Utilizing a rigorous screening process, and a multifunctional approach to target selection, NMS delivers unparalleled ADC development opportunities with enhanced efficacy and safety. See our pipeline.
Proprietary chemical collection
- 1000+ granted patents/applications
- 310+ published papers since 2004
- 100k +compounds cytotoxic and targeted agents
- 20k+ compounds profiled in cells
- Available safety and efficacy studies
Integration and Optimization with Cross-disciplinary approaches
Bioinformatic capabilities to optimize the development of ADCs.
This involves using multifunctional analysis and drawing on extensive internal knowledge to determine the best combinations of targets, payloads, and linkers to deliver the best ADC for any "given" target
NMS’s unique fully integrated approach is a strategic advancement in the ADC field
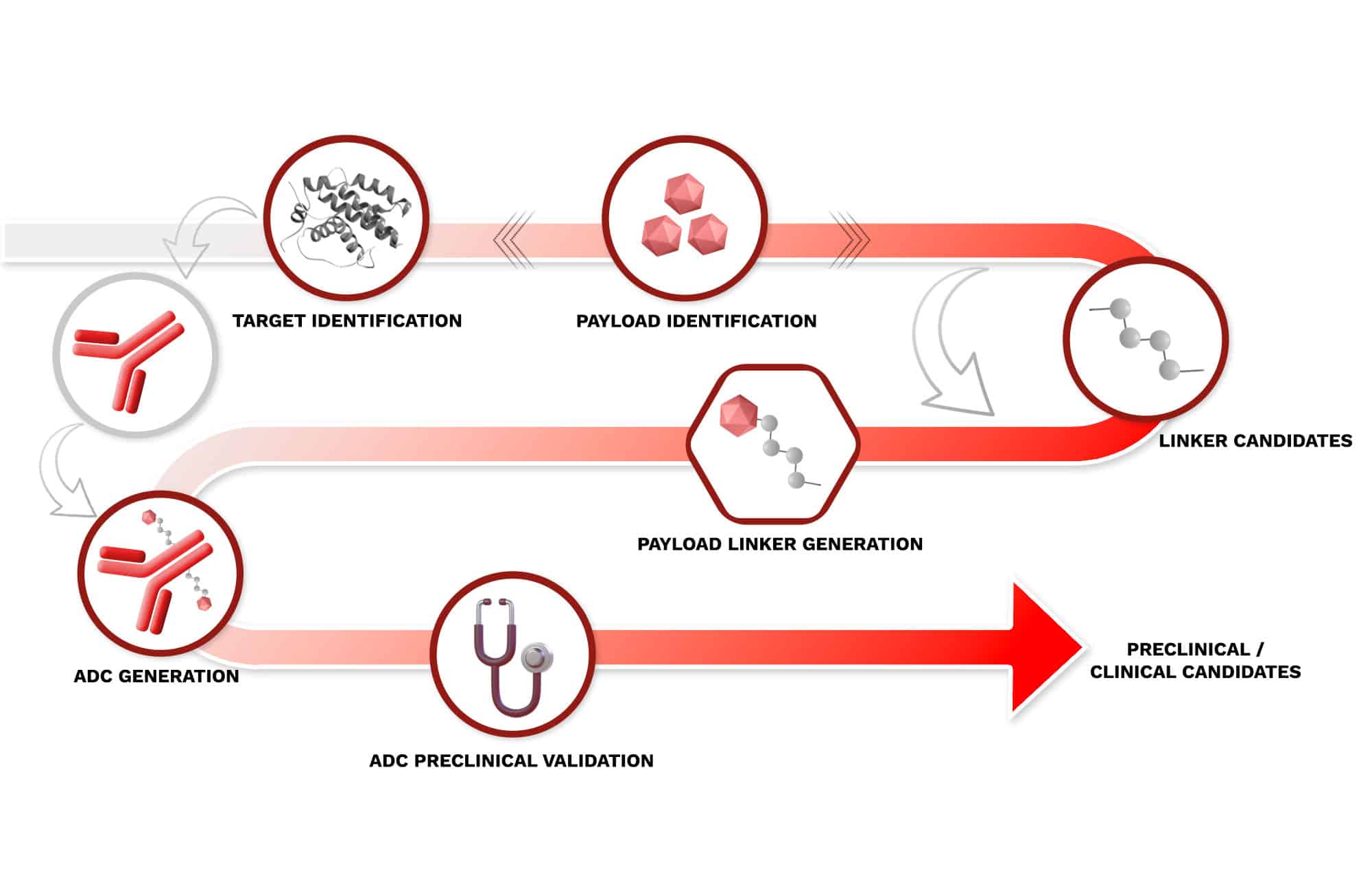
Target Identification
Starting from payload(s) features, a multidimensional approach including tumor sensitivity, medical need and competition drives the selection of the best suited target(s) for each payload-linker.
Payload Identification
Starting from proprietary historical data and chemical collections, payloads are prioritized based on proliferation, safety and efficacy data, together with chemical feasibility and diversity.
Payload Linker Generation
Payloads prepared with different linkers are prioritized based on chemical stability and payload release.
ADC Generation
Developing and prioritizing ADCs involves a rigorous evaluation of multiple critical parameters including Drug Antibody Ratio (DAR), aggregation, binding, antiproliferative activity in target (+) and target (-) cells, bystander effect, immunogenic cell death and plasma stability.
ADC Preclinical Validation
The process involves extensive testing in both in vitro and in vivo models to assess key aspects of preclinical validation such as efficacy in CDX and PDX models, and pharmacokinetic properties.
Target Identification
Starting from payload(s) features, a multidimensional approach including tumor sensitivity, medical need and competition drives the selection of the best suited target(s) for each payload-linker.
Payload Identification
Starting from proprietary historical data and chemical collections, payloads are prioritized based on proliferation, safety and efficacy data, together with chemical feasibility and diversity.
Payload Linker Generation
Payloads prepared with different linkers are prioritized based on chemical stability and payload release.
ADC Generation
Developing and prioritizing ADCs involves a rigorous evaluation of multiple critical parameters including Drug Antibody Ratio (DAR), aggregation, binding, antiproliferative activity in target (+) and target (-) cells, bystander effect, immunogenic cell death and plasma stability.
ADC Preclinical Validation
The process involves extensive testing in both in vitro and in vivo models to assess key aspects of preclinical validation such as efficacy in CDX and PDX models, and pharmacokinetic properties.
NMS’s portfolio of payload linkers: expanding the ADC landscape enhancing the effectiveness and precision of cancer treatment

NMS established a unique platform of cytotoxic and targeted payload linkers with peculiar features to develop the best ADC for any given target or tumor type:
NMS-P945 is a duocarmycin-based payload linker, acting as a DNA damaging agent and demonstrating strong bystander effect, high in vivo efficacy with cured mice and good safety profile suitable for chemo resistant highly heterogeneous solid tumors (Mol Cancer Ther (2023) 22 (12): 1465–1478).
Next generation anthracyclines are topoisomerase II inhibitors showing outstanding immunogenic cell death properties, superior efficacy and safety profile to PNU payload suitable to revert cold into hot tumors (AACR2024 , NMCS_ACS2024 posters).
Novel targeted payloads with diversified mechanisms of action and to be developed with selected targeted antibodies to reach a unique selectivity and increased safety profile compared with current ADCs (WADC-EU 2024 poster).
Target-Payload matching via a multidimensional approach based on individual payload(s) features, tumor settings and market perspectives. Potential for increased selectivity and greater therapeutic window in patients.
See the poster presented at:
– AACR 2024 “A novel platform of diversified cytotoxins and targeted payloads to drive ADC innovation”
– ACS 2024 “Development of 14-amino-anthracyclines as novel payloads for ADC production showing potent anti-tumor activity and improved safety profile”
– WADC Europe 2024 “A payload-linker generating machine to quickly move from small molecules to characterized tool ADCs and PDCs”
– WADC San Diego 2024
- Poster “NMS ADCs platform: innovation by design”
- Presentation by Barbara Valsasina “Showcasing NMS’s Portfolio of Cytotoxins & Targeted Payloads to Drive ADC Innovation”